Together for
Advanced Therapies
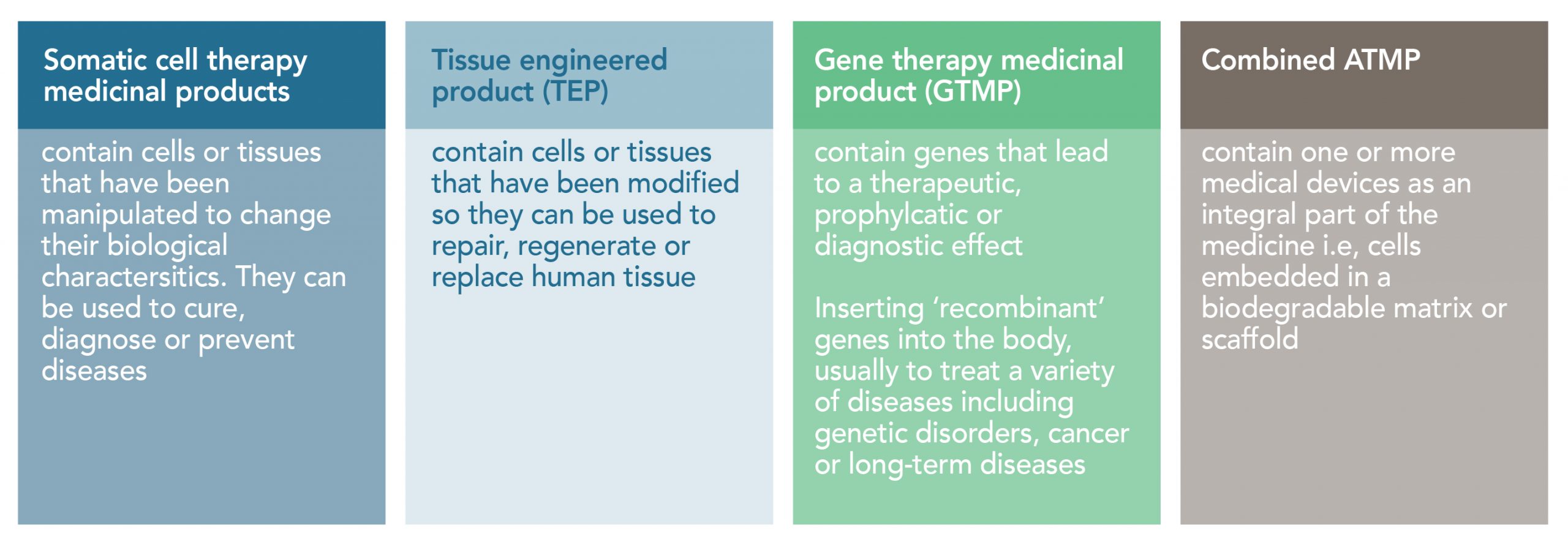
Different types of ATMPs
Advanced therapy medicinal products or ATMPs are medicines for human use that are based on genes, tissues or cells. They offer groundbreaking new opportunities for the treatment of life-threatening diseases where one or limited doses have a curative effect.
There is a significant increase in investments in this area which is largely driven by advancements in science and favorable regulatory support.
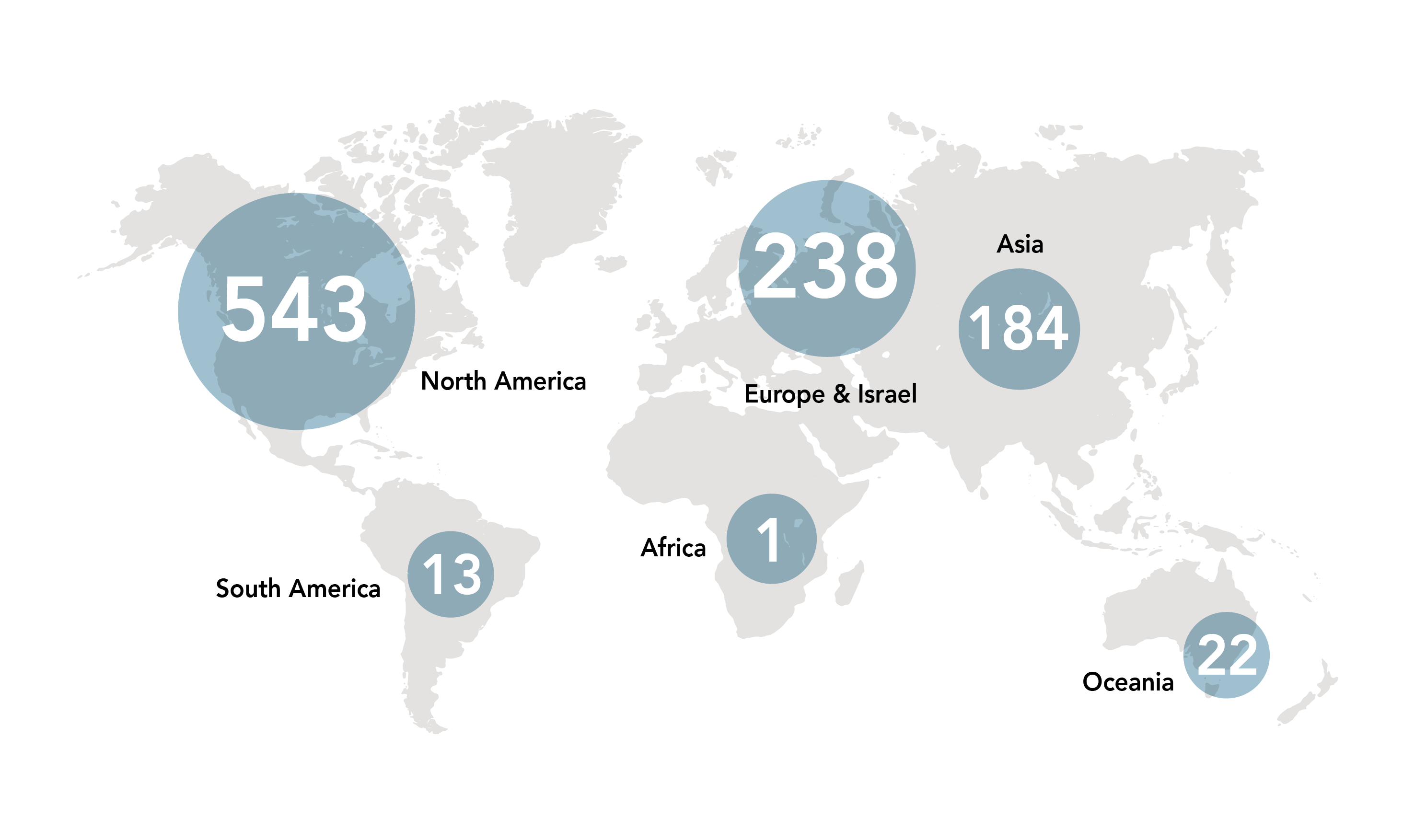
Active Regenerative Medicine and Advanced Therapy Developers Worldwide
Ongoing Regenerative Medicine and Advanced Therapy Clinical Trials Worldwide
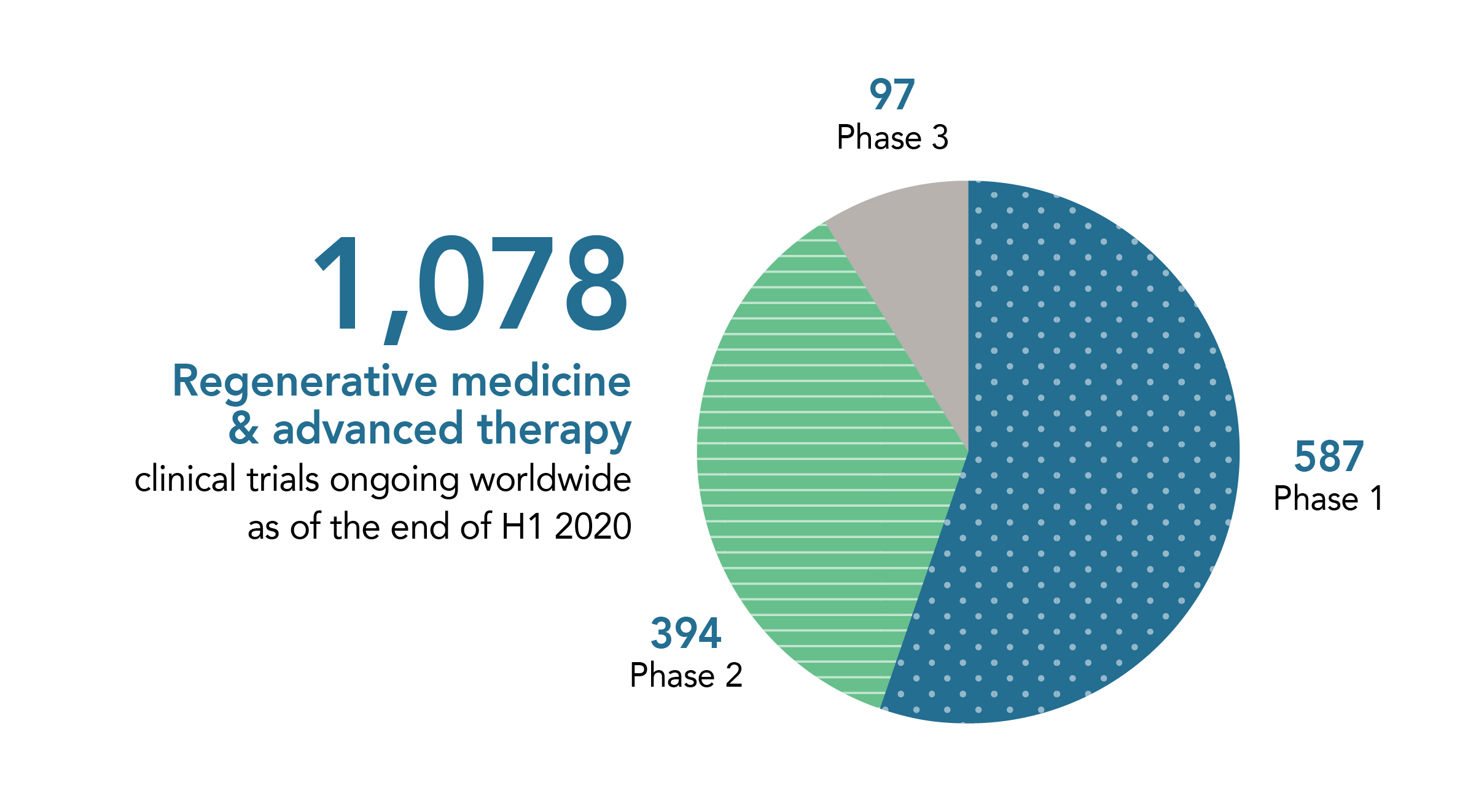
(source: ARM Global Regenerative & Advanced Therapy Medicine Sector Report: H1 2020)
Some of the recent ATMPs that have been granted approvals include Strimvelis, Kymriah, Yescarta, Luxturna and Zolgensma.
Novelty of ATMP makes it the future of medication
Breakthrough treatments resulting from ATMPs allow biopharmaceutical companies to get accelerated regulatory approvals.
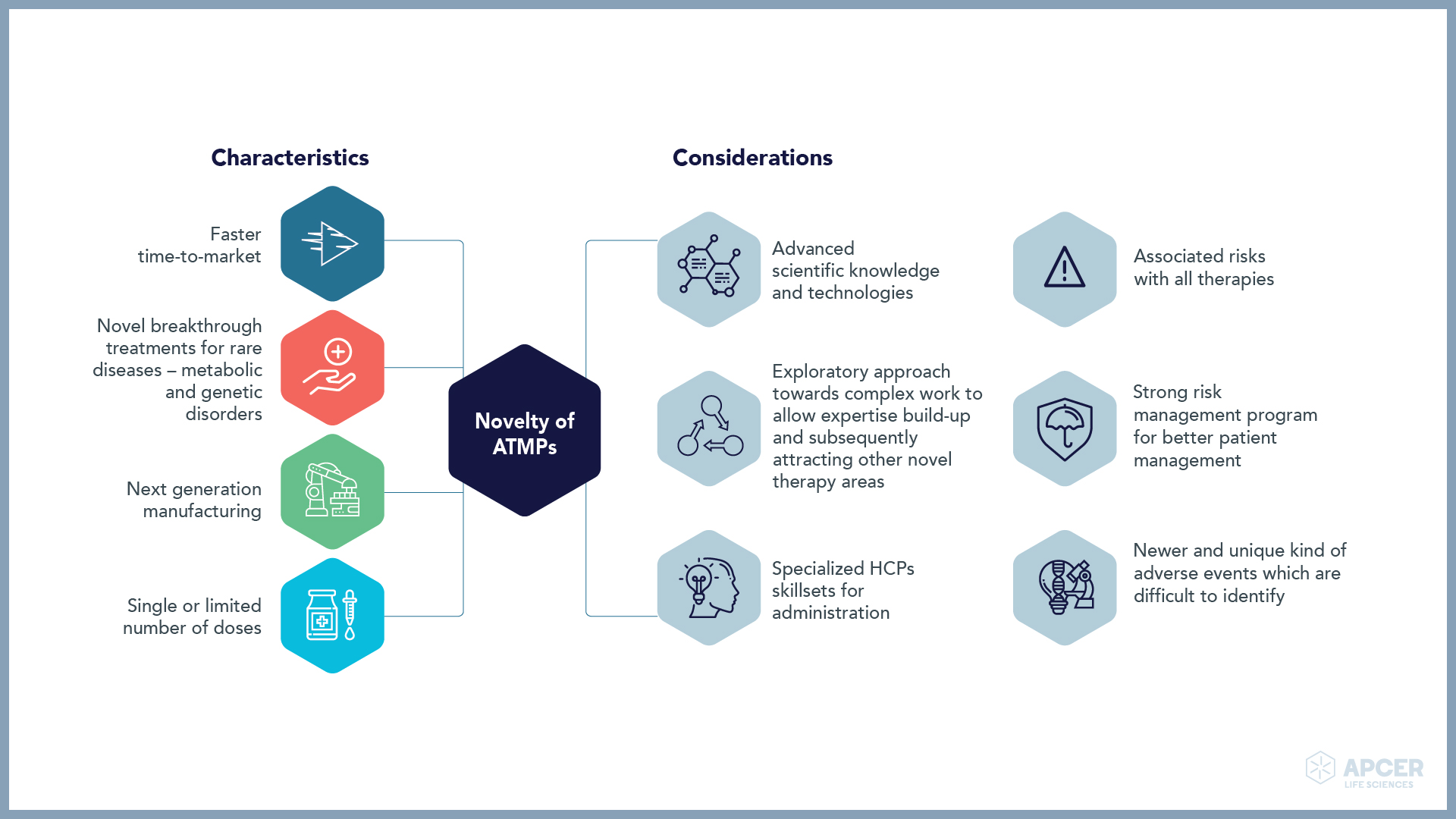
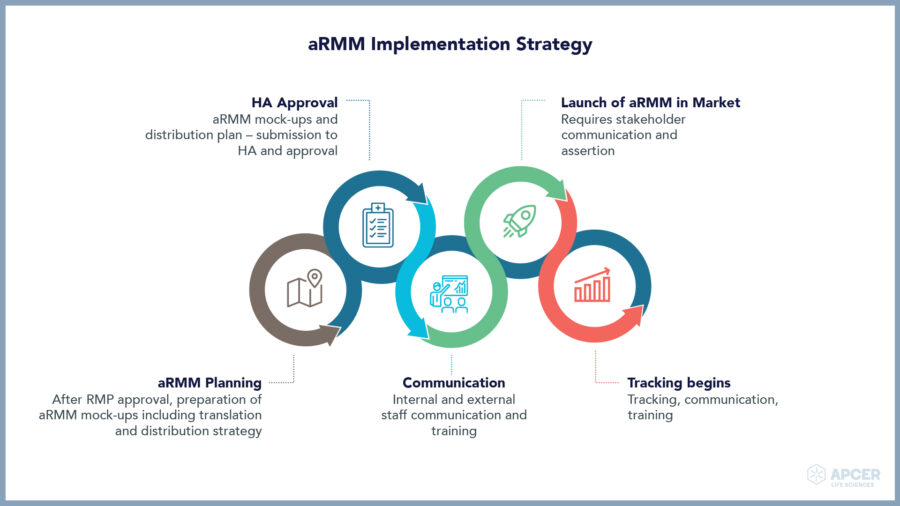
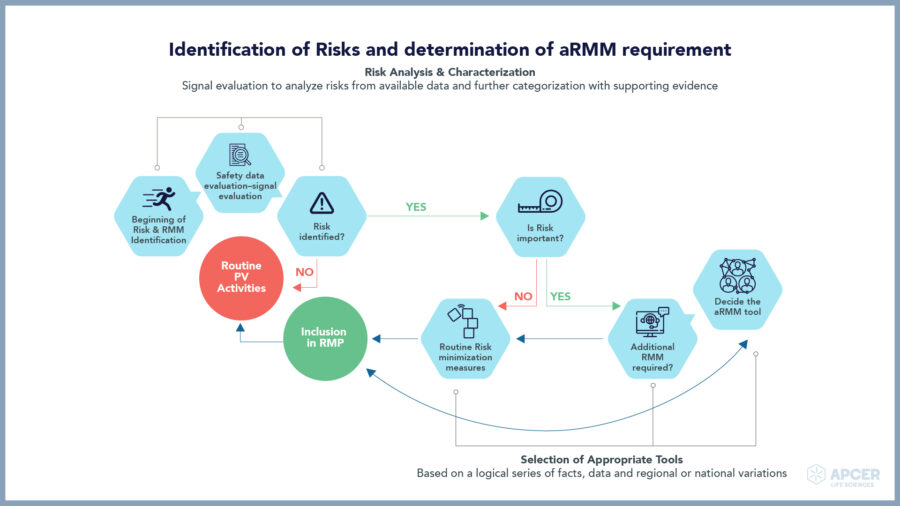
Major safety challenges faced by ATMP companies
ATMP companies need to take a long-term approach towards collection and analysis of safety data for maintaining the overall risk-benefit balance.
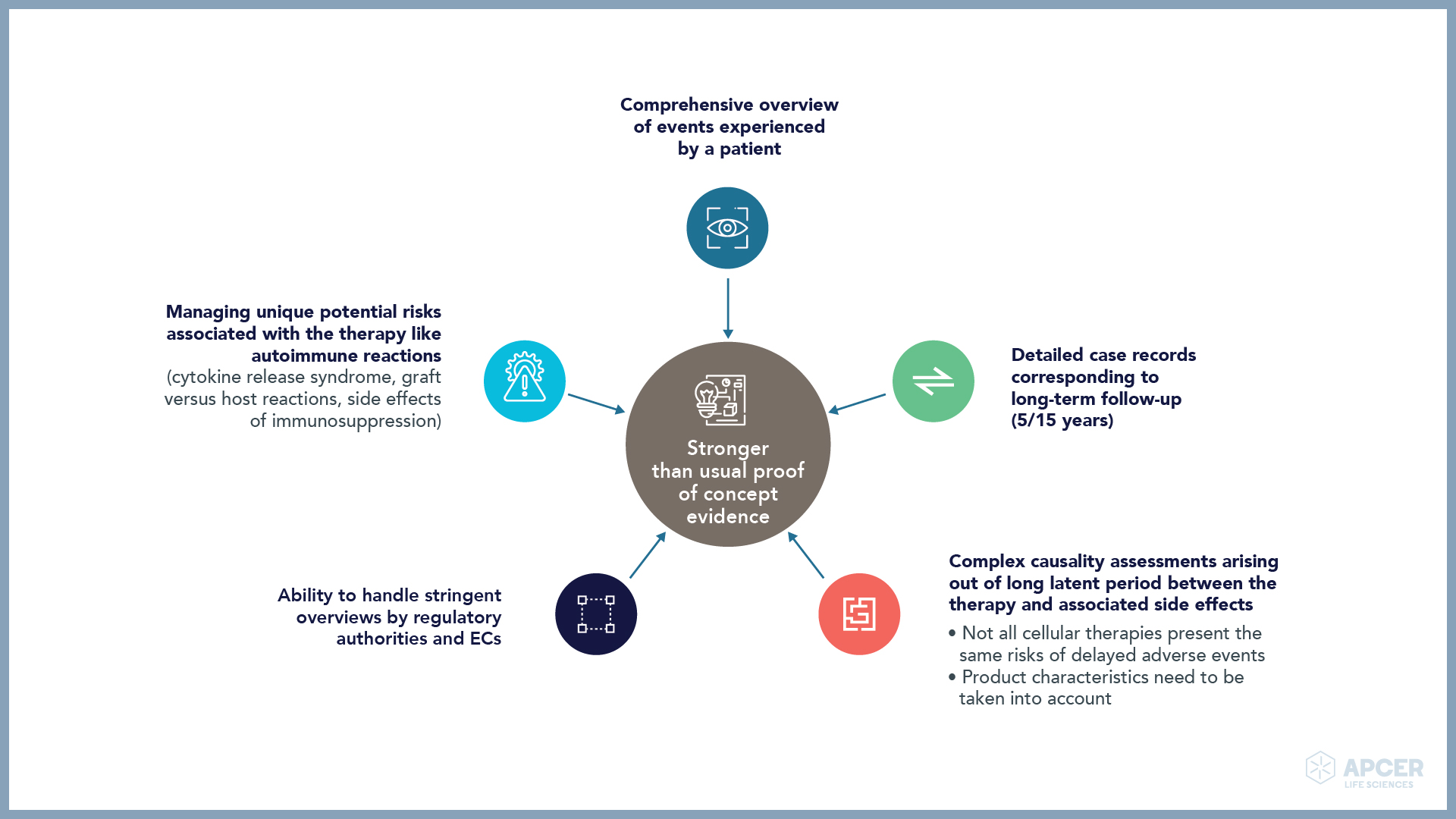
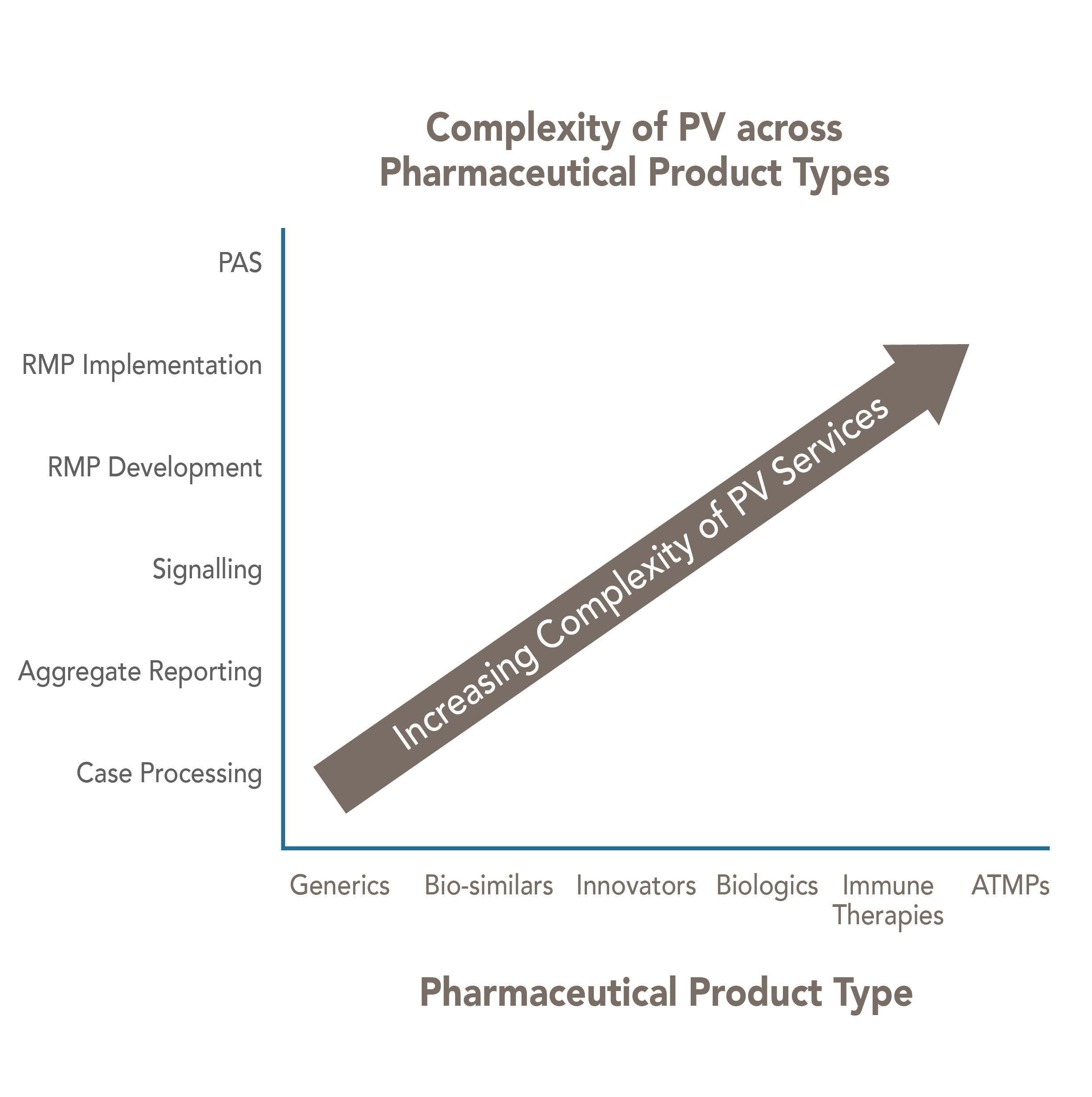
Novel therapeutic products require a Specialized Safety Expert
Most of the ATMP companies are offering one to two products. These companies are pre-dominantly focused on development and successful commercialization of drugs. However, new technologies and product platforms create uncharted regulatory paths for drug development and marketing authorization.
They require a Safety partner who brings specialized skills to help them strategize and manage their complex Safety requirements throughout their ATMP life cycle.
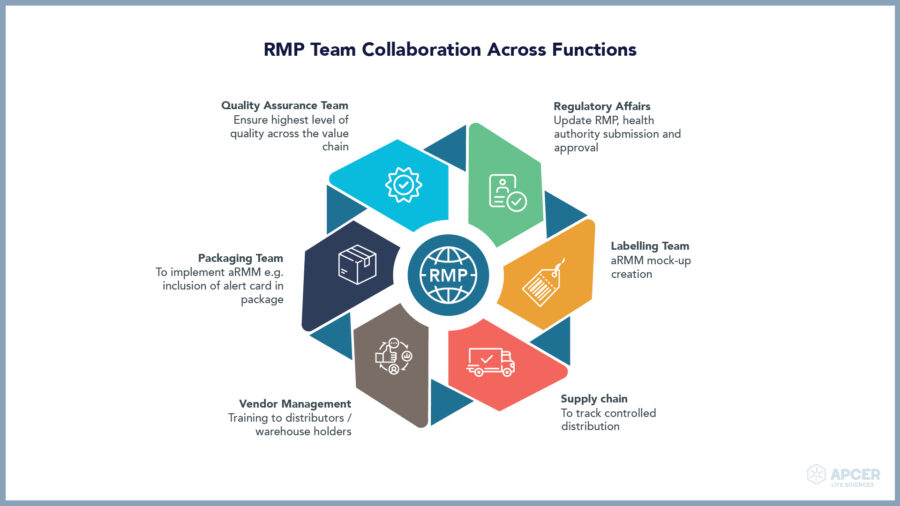
APCER Life Sciences – Specialized Safety Partner for ATMP Life Cycle Management
We are your trusted Safety Partner for full life cycle management across risk minimization documentation and planning, clinical trials, submission phase, post-approval planning and post-approval.
Our experienced Safety team is proficient in:
- Complex case processing and long term follow ups
- Large risk management and REMS programs
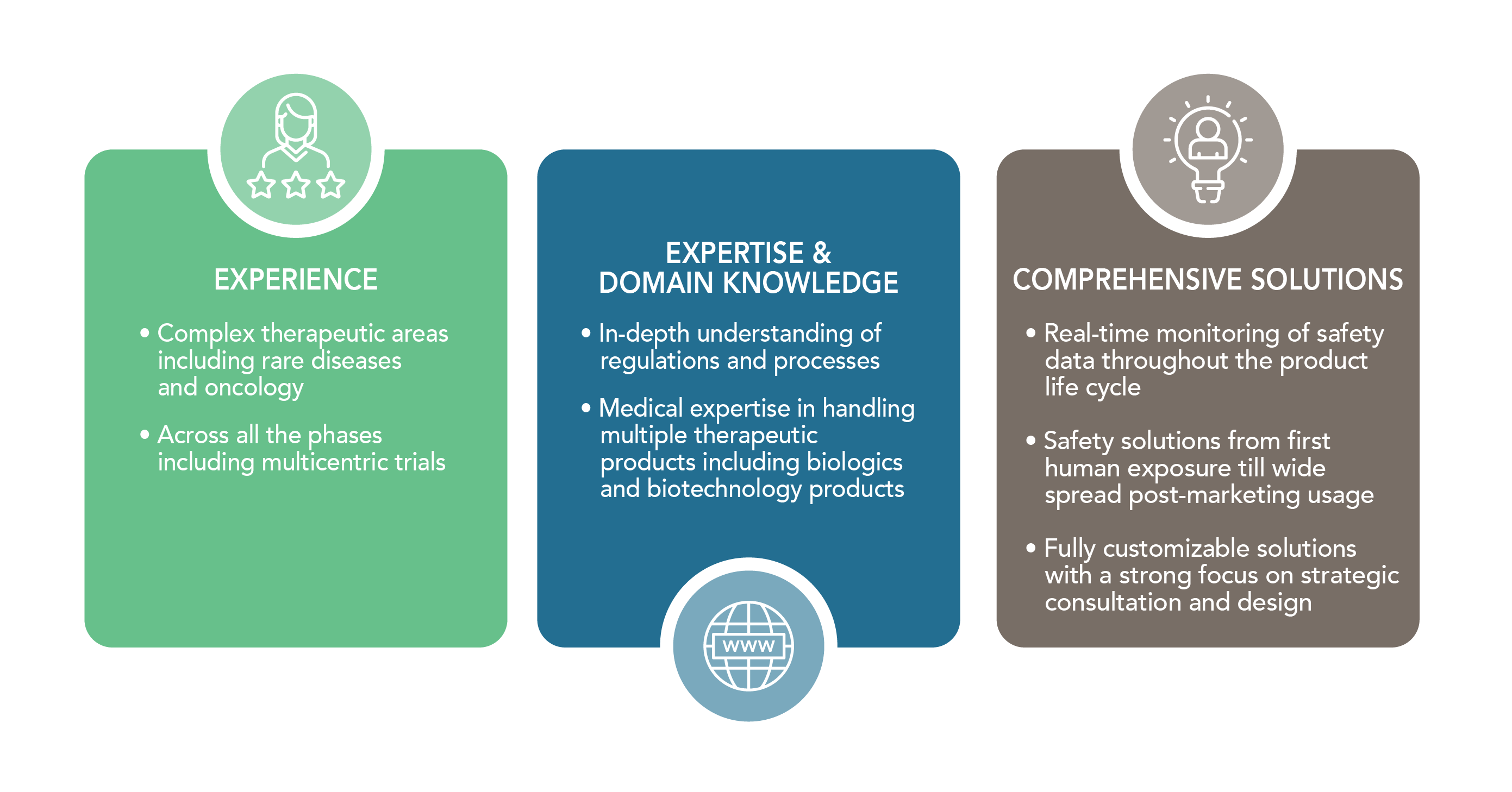
- Multi-therapeutic area expertise including rare diseases and oncology
- Regulatory support including BLAs and expedited submissions
- Medical writing for all clinical trial documentations across the ATMP life cycle
We will partner with you to navigate the uncharted Safety landscape within the ATMP life cycle.
APCER Advantage