Insights
November 5, 2024
Navigating FDA Type B Meetings: Case Study Overview APCER Life …
October 7, 2024
Securing Type C meeting with FDA for Oncology Trial: Case …
September 13, 2024
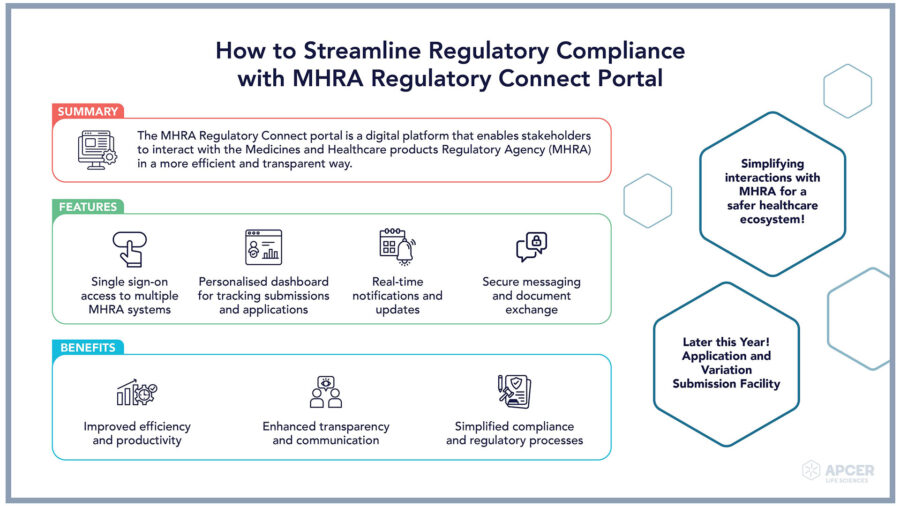
MHRA Regulatory Connect Portal: An Overview The MHRA Regulatory Connect …
September 11, 2024
Submission of Common Technical Document Modules: Case Study Overview A …
August 30, 2024
Managing Global and Local Literature Surveillance: Case Study Overview Client …
July 30, 2024
With an aggressive go-live target and a need for experienced …
June 27, 2024
A global pharmaceutical company leveraged our Scientific Writing team’s expertise …
June 17, 2024
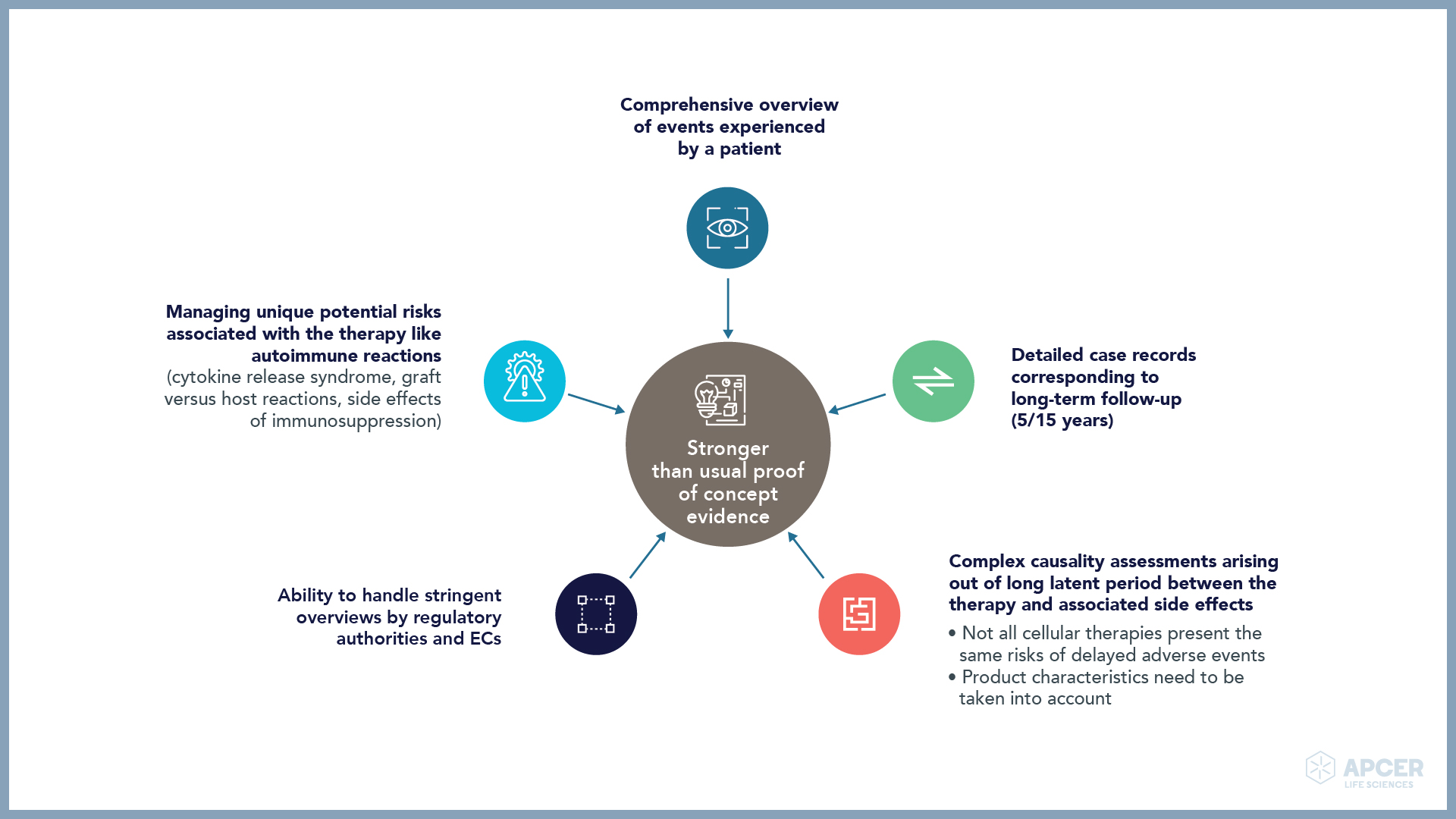
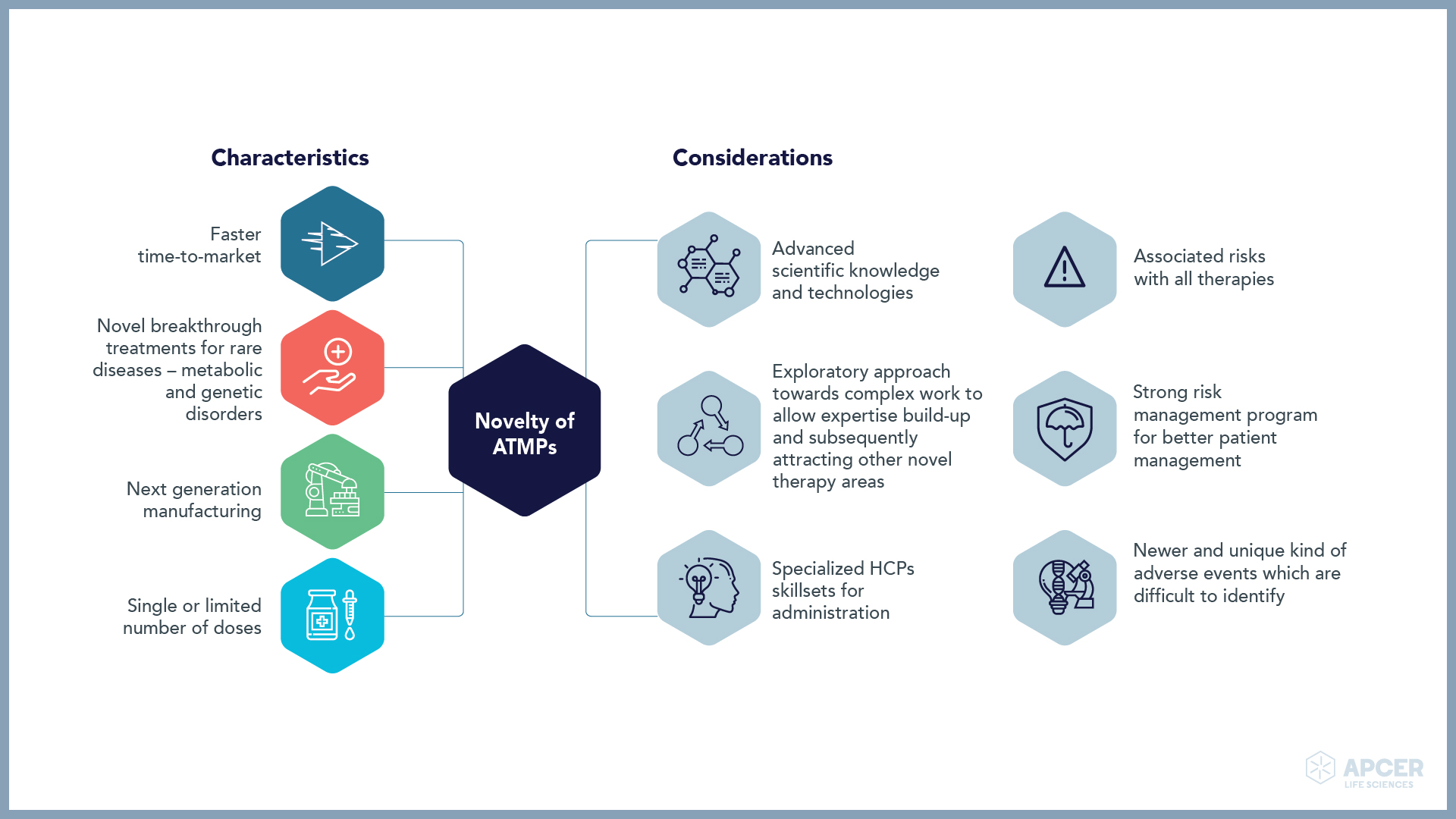
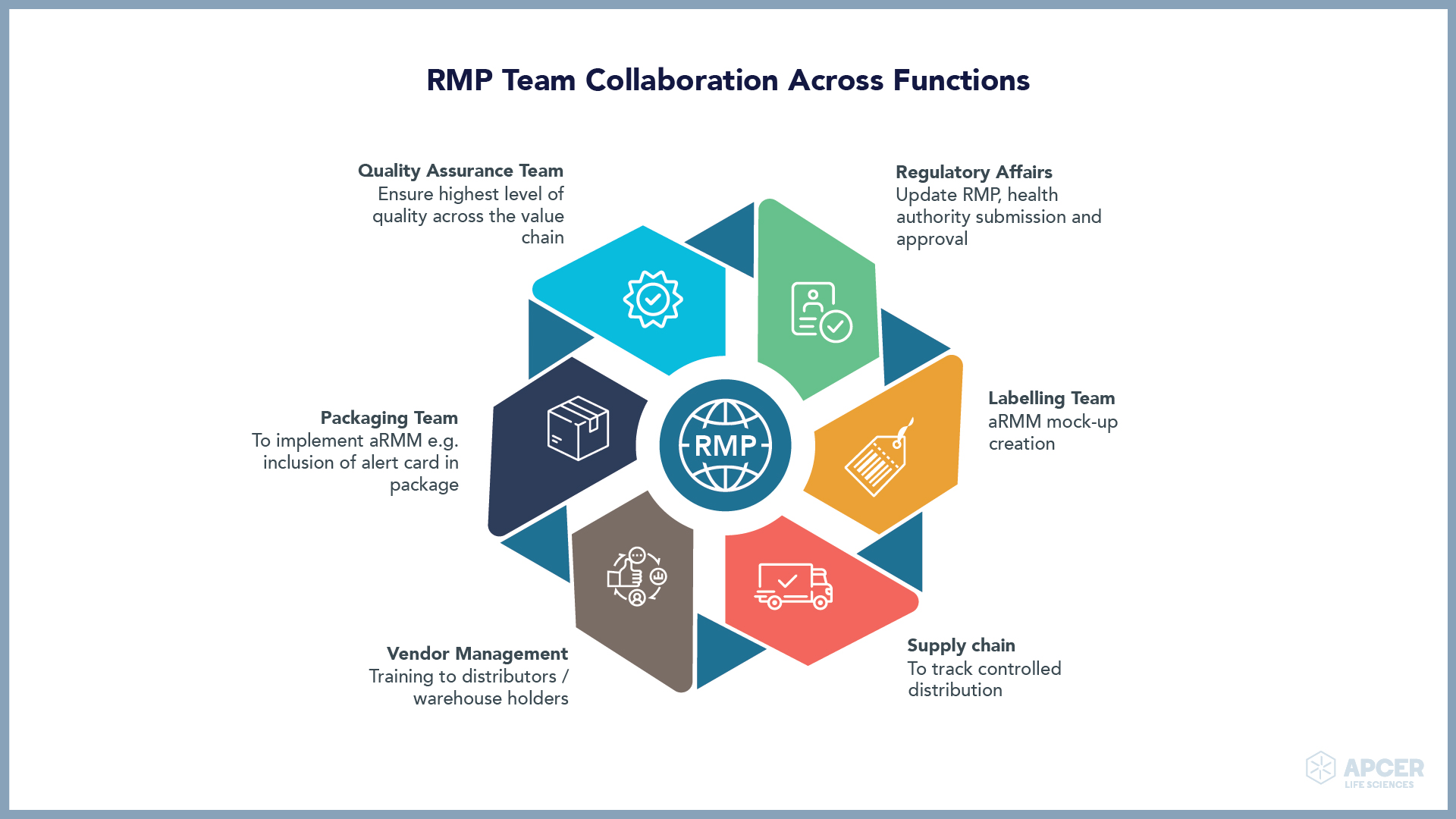
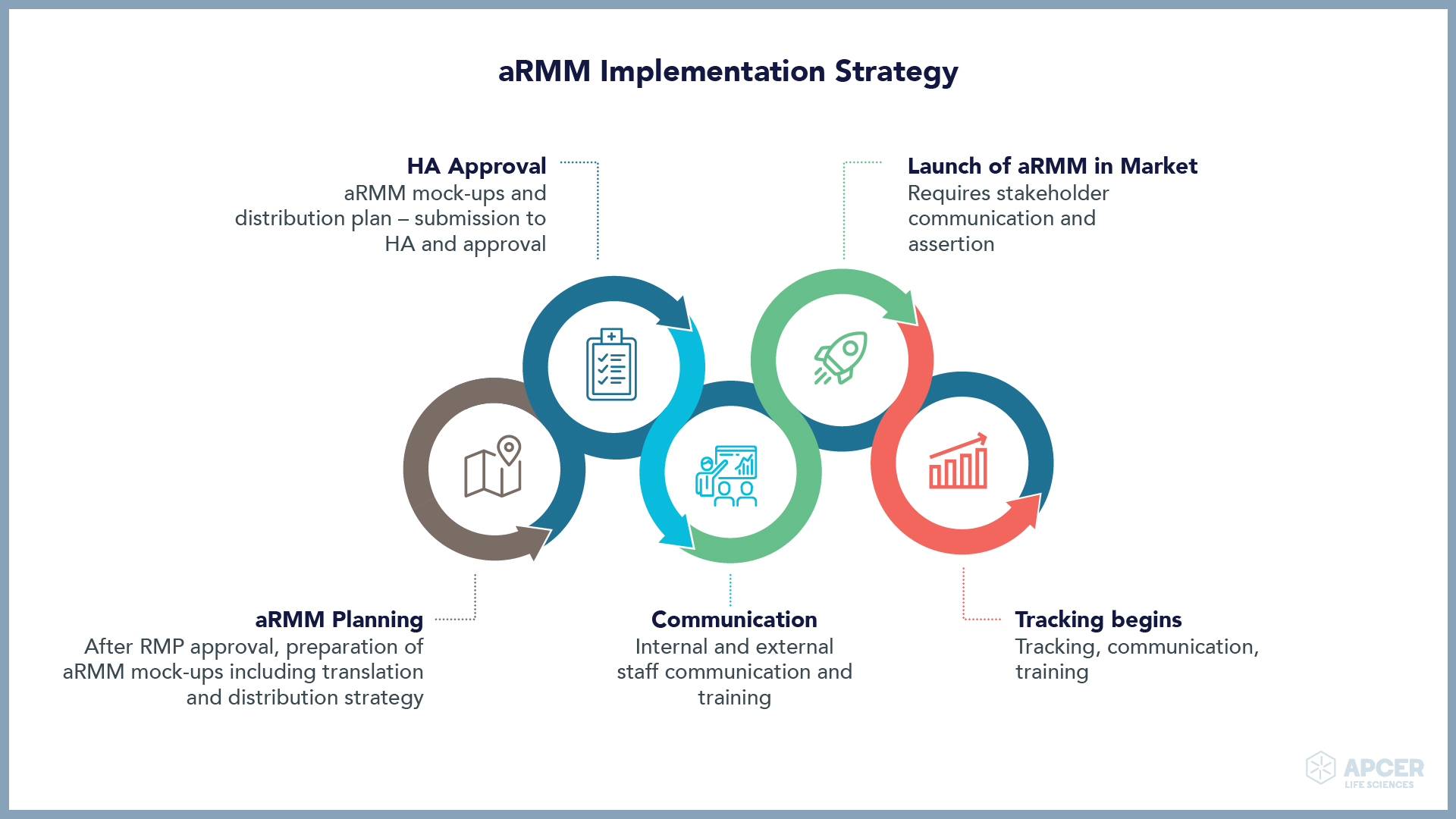
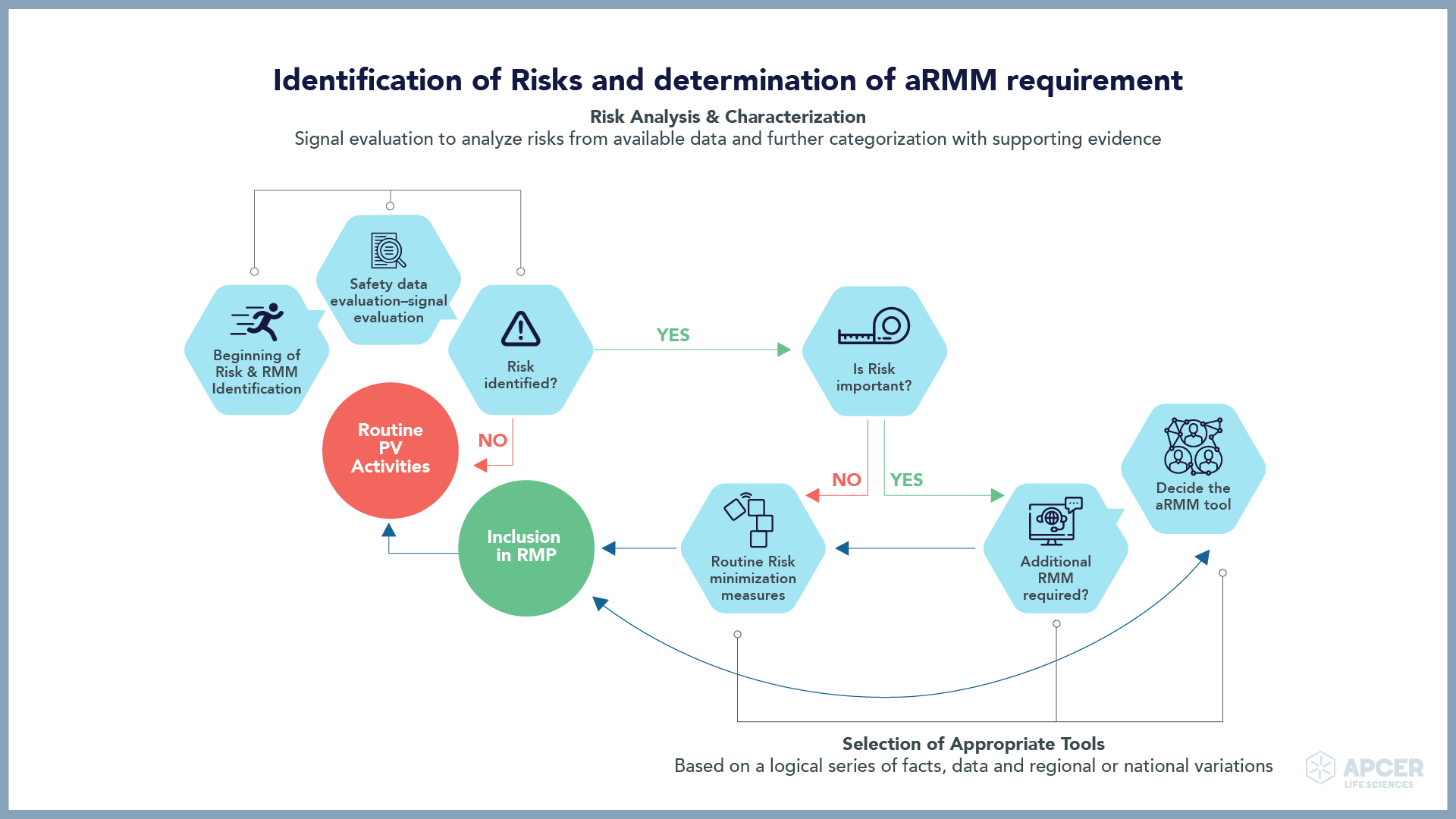
Identifying and managing safety concerns, implementing risk minimization measures (RMMs), …
April 30, 2024
Compiling Large Volumes of Real World Data and Creating a Report with Appendices for a Global Client
Our Medical Writing (MW) team demonstrated exceptional expertise and dedication …
April 5, 2024
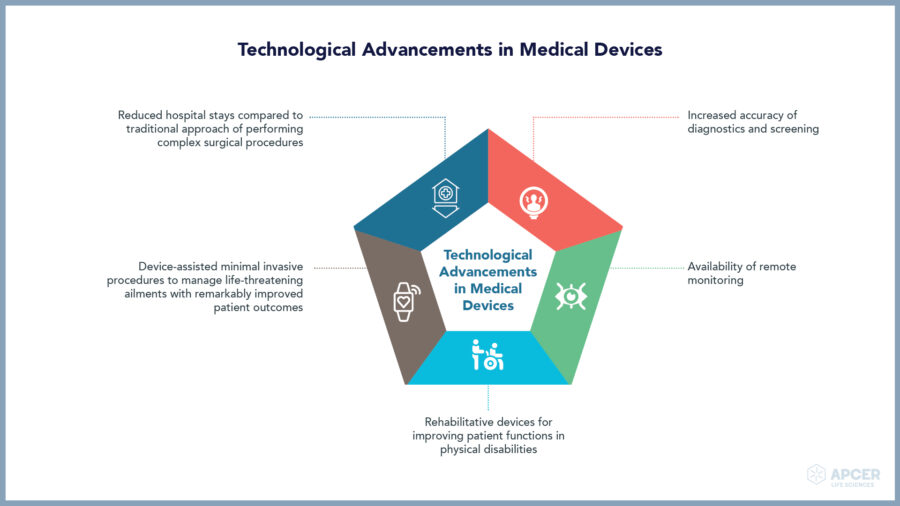
A leading device manufacturer faced challenges complying with complex device …
February 19, 2024
September 21, 2023
May 3, 2023
CMC writing is a standardized scientific and technical writing process …
March 6, 2023
A drug product transitions from different phases throughout its life …
February 22, 2023
December 6, 2022
With the ever-growing knowledge of science and application of advanced …
September 30, 2022
Managing Medical information (MI) services in-house is a major challenge …
September 2, 2022
Our Medical Writing team helped the client in updating and …
August 5, 2022
Ms. Shellie Cholke, Vice President – Sales talks about three …
June 20, 2022
APCER Life Sciences provides the right expertise and support required …
June 16, 2022
Our Integrated Pharmacovigilance operating model offers choice of best-in-class databases …
May 18, 2022
We supported a large biopharma company in preparing manuscript/narrative review …
April 21, 2022
APCER life sciences through its oncology expertise helped the pharma …
April 26, 2021
April 23, 2021
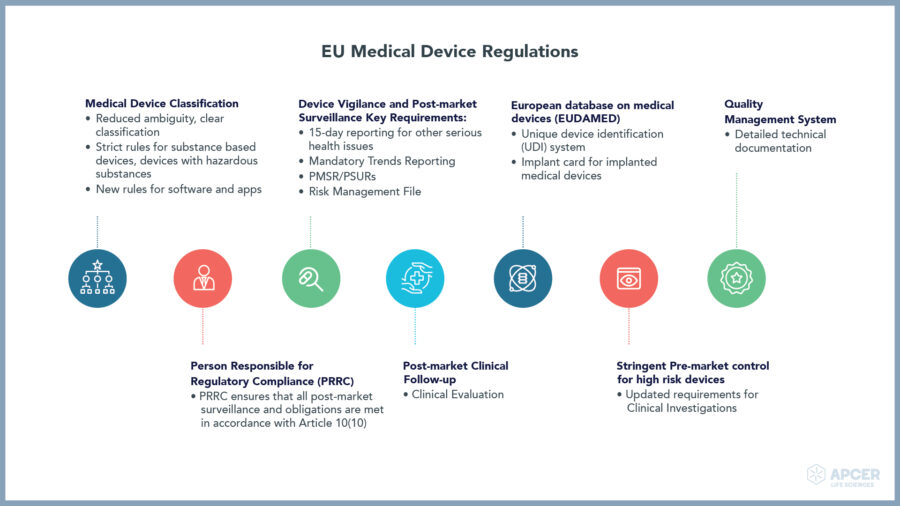
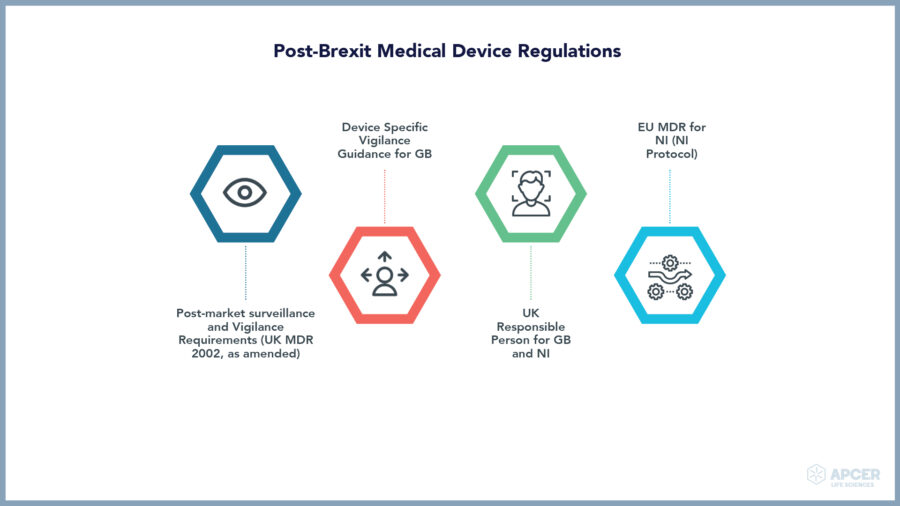
Rapidly changing regulations in the European Union are making regulatory …
April 23, 2021
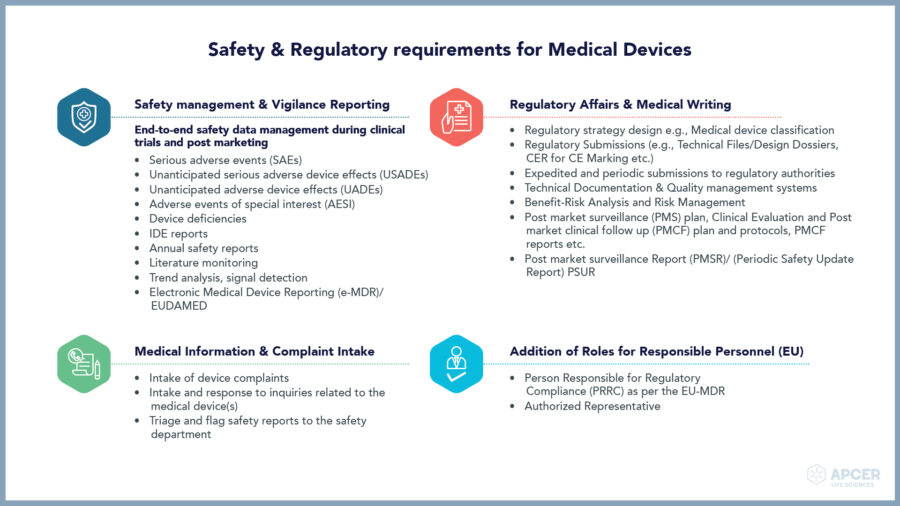
The EU and UK regions have stringent Pharmacovigilance and regulatory …
April 5, 2021
March 9, 2021
March 8, 2021
APCER’s Pharmacovigilance (PV) team supported a client’s expansion into new …
March 8, 2021
APCER’s Pharmacovigilance (PV) team helped a biotech pharmaceutical company with …
March 8, 2021
March 8, 2021
APCER helped a leading generics company to streamline its PV …
March 8, 2021
We helped a large pharmaceutical company successfully transition its pharmacovigilance …
March 8, 2021
Our Medical Writing team helped the client in handling e-submission …
March 8, 2021
APCER helped a global pharmaceutical company in establishing a collaborative …
March 8, 2021
APCER Life Sciences supported one of the biopharma companies in …
March 8, 2021
APCER Life Sciences supported a leading generic pharma company with …
March 8, 2021
The Regulatory Affairs team at APCER Life sciences helped the …
March 8, 2021
Read how our Quality Assurance team helped a pharma company …
March 8, 2021
APCER’s Pharmacovigilance (PV) team provided PV services right from set …
March 8, 2021
We helped a biopharma company to set up end-to-end Quality …
March 8, 2021
APCER Life Sciences helped develop the strategy and provided medical …
March 8, 2021
Our medical monitoring team with experience and expertise in early …
January 13, 2021
January 12, 2021
Ms. Jeanne Schow, Vice President & US Head of Business …
January 10, 2021
January 10, 2021
January 10, 2021
January 10, 2021
APCER Life Sciences provides the expertise, robust processes and infrastructure …
January 10, 2021
January 6, 2021
APCER Life Sciences provides 24×7 fully customizable, multi-channel and multi-lingual …
January 6, 2021
We offer end-to-end regulatory consulting and execution support services to …
January 6, 2021
December 11, 2020
Dr. Vineet Kacker, Managing Director & Global Technical Head talks …
November 5, 2020