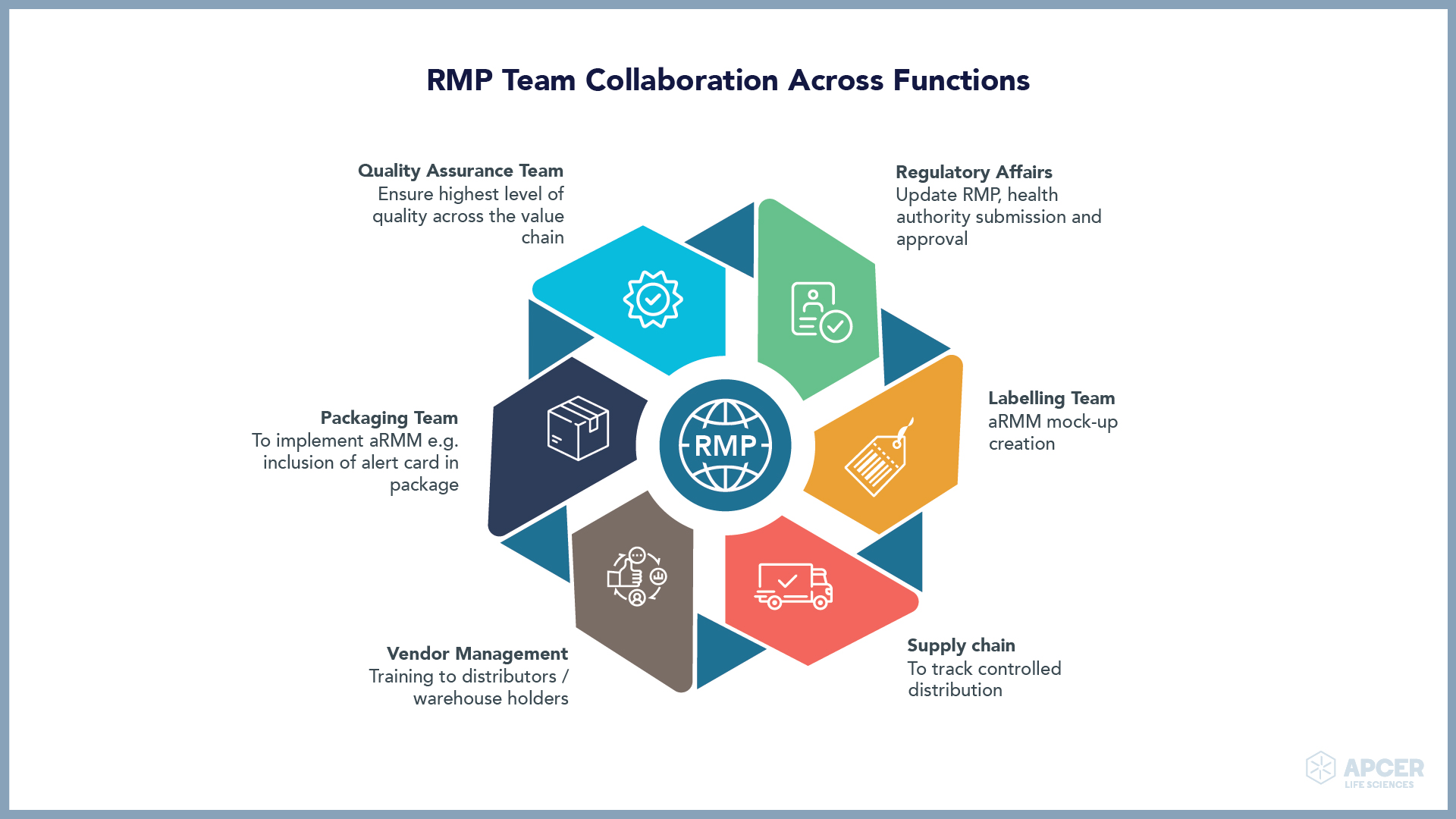
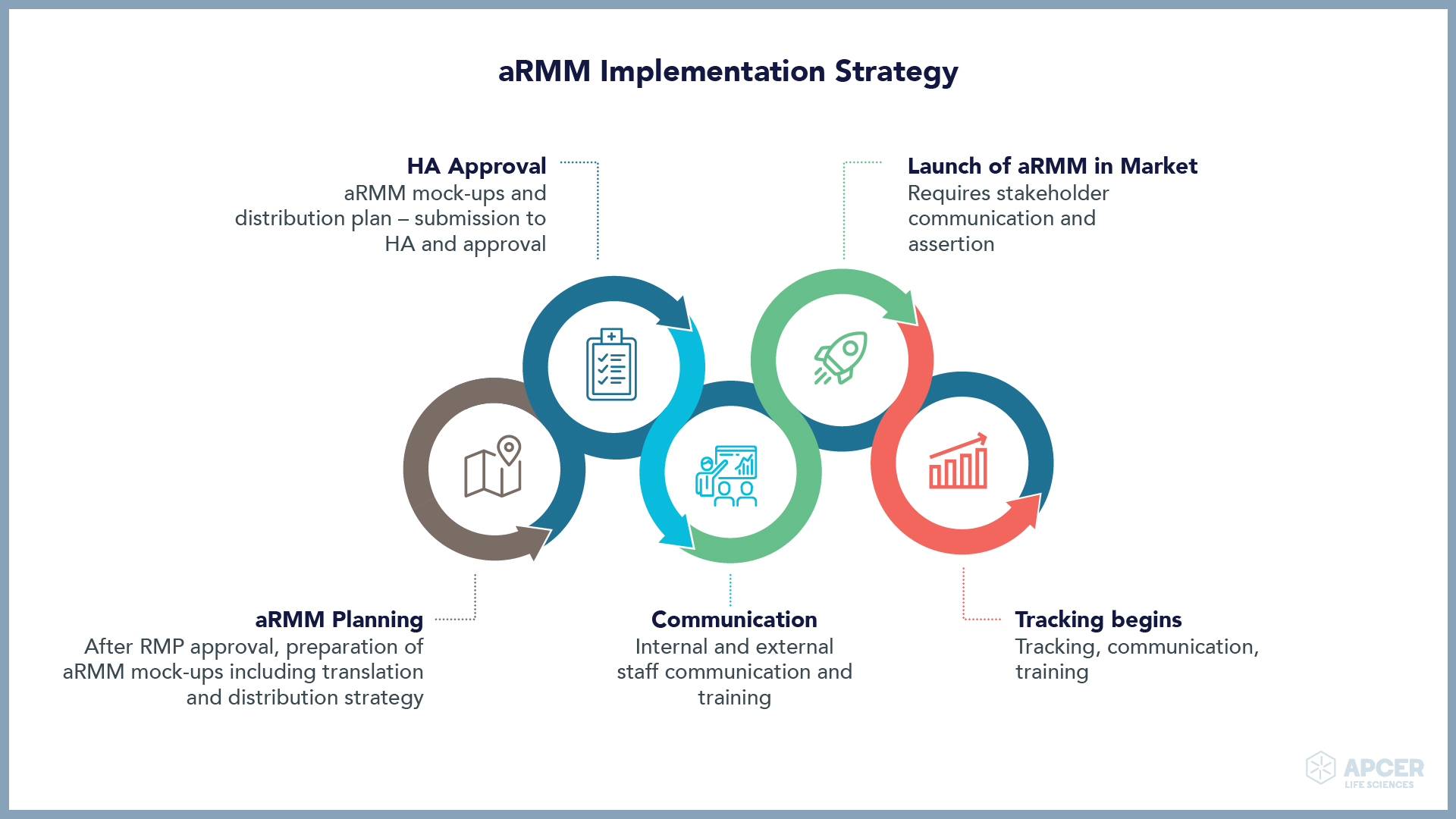
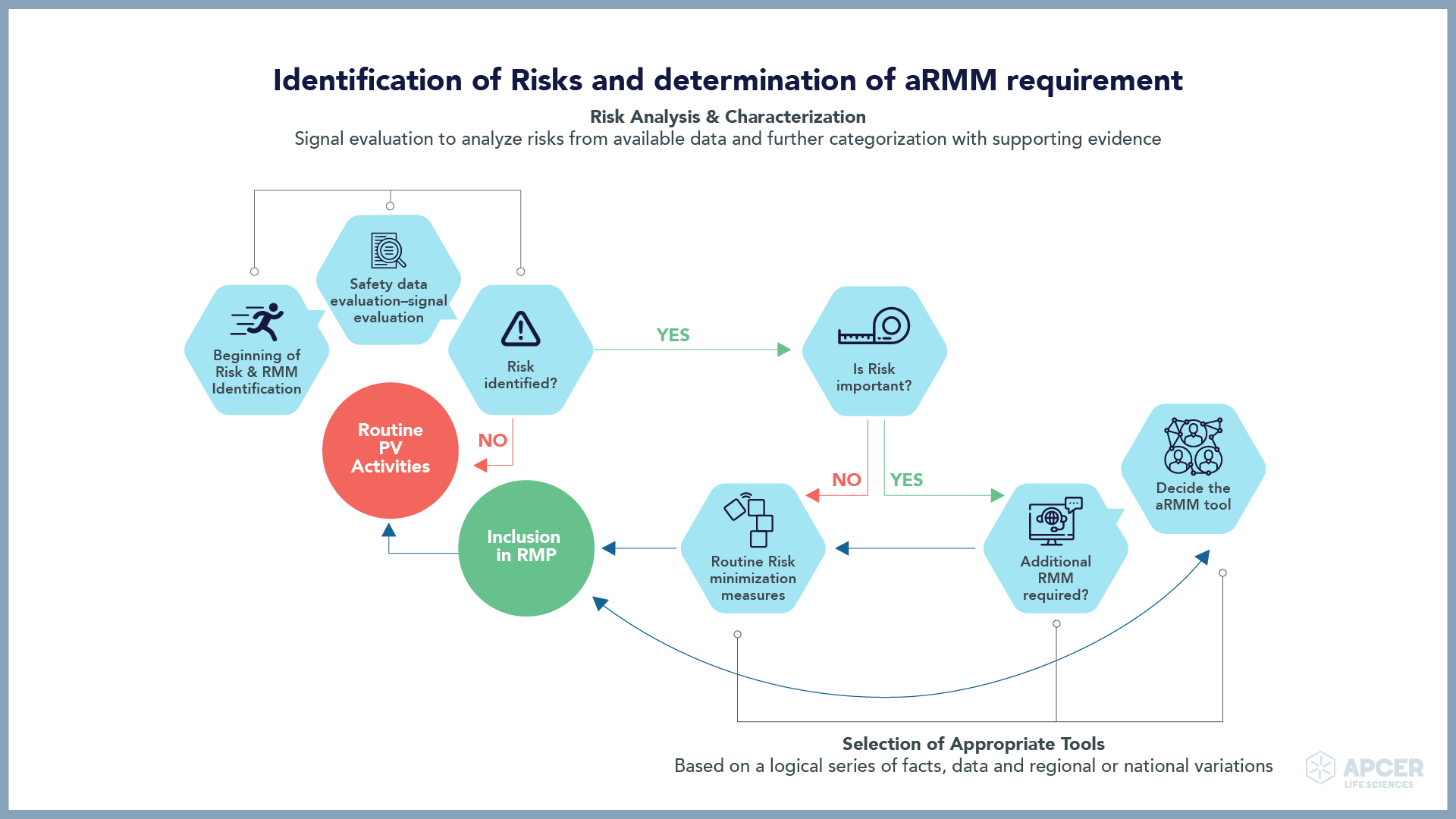
Pragmatic Approaches
March 8, 2021
APCER’s Pharmacovigilance (PV) team supported a client’s expansion into new …
March 8, 2021
APCER’s Pharmacovigilance (PV) team helped a biotech pharmaceutical company with …
March 8, 2021
APCER helped a leading generics company to streamline its PV …
March 8, 2021
We helped a large pharmaceutical company successfully transition its pharmacovigilance …
March 8, 2021
APCER helped a global pharmaceutical company in establishing a collaborative …
March 8, 2021
APCER Life Sciences supported one of the biopharma companies in …
March 8, 2021
APCER Life Sciences supported a leading generic pharma company with …
March 8, 2021
The Regulatory Affairs team at APCER Life sciences helped the …
January 12, 2021
Ms. Jeanne Schow, Vice President & US Head of Business …