Assurance services for safety of vaccines
Vaccines are among the most important medical interventions for prevention of illness and life-threatening conditions especially in emergency situations like the ongoing COVID-19 pandemic.
The general acceptance and tolerance for any adverse events following vaccination is low because vaccines are generally administered to healthy persons to prevent disease. Thus, a higher standard of safety is expected for immunizations compared to treatment modalities.
However, vaccines, like other pharmaceutical products, are not completely risk-free and adverse events do occur which can range from minor side-effects to more severe reactions.
Vaccine Pharmacovigilance
- Ensure the minimization of negative effects to individuals: Vaccine pharmacovigilance aims to detect adverse events early to trigger accurate risk assessment and appropriate response (risk-management) to the problem.
- Lessen the potential negative impact on immunization programs: This lowers tolerance for risks from vaccines translates into a greater need to detect and investigate any adverse event following immunization (AEFI) than is generally expected for other pharmaceutical products.
Vaccines are regulated right from development, to licensure and, to final use. National regulatory authorities play an important role in the entire process. Therefore, implementation of proven safety measures is required:
- Capacity to handle a large number of adverse event reports in a short period of time and collection of adverse events following immunization (AEFI).
- Early assessment of the potential connection between an event and the vaccine administered.
- Assessment of vaccine failures or reversal of virulence for live attenuated vaccines.
- Accuracy and efficiency to identify, assess and analyze signals.
- Understanding complex global regulatory requirements for vaccine licensurefor
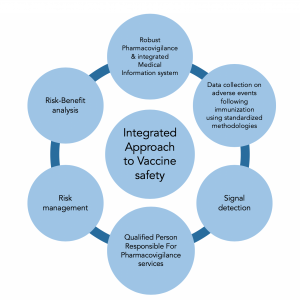
An approach to Vaccine Pharmacovigilance
APCER Life Sciences, as your partner, understands the nuances of planning and execution in addressing vaccine safety needs.
We partner with companies by:
- Leveraging cumulative experience of over 12 years in complex therapeutic areas including vaccines (Outbreak, Travel, Infectious diseases)
- Travel vaccines (Typhoid, Cholera, Measles, Diptheria)
- Outbreak vaccines (Anthrax, Botulism, Small poax)
- Developmental Products (Chikunguya, Zika, Anthrax, Covid-19)
- Setting up a robust Medical Information & Pharmacovigilance integrated system
- Quality-driven integrated advanced software solutions for management & collation of large number of serious & non-serious adverse events in a short period of time.
- Combining strong medical assessment and expertise with efficient operational processes for handling complexities in case processing, follow-ups and aggregate reporting.
- Developing Standard Operating Procedures, managing study configuration specifically to therapeutic products like vaccines under aggressive timelines
- Bringing together experienced teams across all phases including global multicentric trials for multiple therapeutic areas
- Supporting regulatory submissions of reports via accepted pathways to FDA and other Regulatory Authorities.
- Scaling up quickly to manage unique situations such as pandemics/epidemics and product recalls.
- Robust Vaccine Adverse Event Reporting System (VAERS) setup for collecting vaccine related information to maintain vaccine safety and to monitor potential rare vaccine associated side effects.
| Vaccine | Category |
| Smallpox vaccine | Anti-viral |
| Anthrax vaccine | Anti-bacterial |
| Japanese encephalitis vaccine | Anti-viral |
| Typhoid vaccine | Anti-bacterial |
| Cholera vaccine | Anti-bacterial |
| Influenza A (H1N1, H5N1 & H3N2) | Anti-viral |
References: 1. WHO. Int/health-topics/coronavirus 2. Centers for disease Control and prevention 3. U.S. Food & Drug Administration
